RG1: Cell Bioprocesses for Advanced Therapies
The “Cell Bioprocesses for Advanced Therapies” RG works at the interface between cell biology/biochemistry advancing biological products required for precision medicine approaches. RG1 develops tools and technologies to support cell and gene based therapies (ATMPs), by bringing biologically relevant phenomena into applications, developing GMP-compatible processes for pharmaceutical industry, and establishing human-cell based in vitro models for pre-clinical research.
Mastering cell physiology of human cells (primary cultures of liver and cardiac cells), adult/pluripotent stem cells and continuous cell lines, allows RG1 to rationally design bioprocesses for optimal cell growth/differentiation and/or maintenance and improved productivity, quality and functionality of cell based products.
Additionally, RG1 comprises molecular biology expertise, handling expression systems to generate novel constitutive viral vector producer cell lines and develop cell-based sensors to assess viral products quality. These products have an enormous potential for drug discovery and therapy and enable the development on in vitro human models for infection and post-infection disease studies contributing to a better understanding disease molecular mechanisms.
Development of analytical assays to characterize cell cultures and their complex products permitting a comprehensive readout of cell function is also central in RG1. Our analytical platforms combine traditional biochemical and chemical methods, bio-imaging tools, cell-based assays, and advanced omics techniques (gen-, transcript-, metabol-, proteo-, glyco- and lipid- omics) based on HPLC, NMR and MS.
We will reinforce our leading position in bioprocess development for ATMPs differentiating our expertise internationally. A special focus will be on the use of these integrative approaches to leverage cancer vaccines into the clinics, in particular within iNOVA4Health TLs Neurosensory and Vision Disorders and Cardio-Metabolic Disorders.
Within the many contributions of RG1 during the period of 2013-2017, three key research lines highlight the Team´s competences and excellence:
1. Robust manufacturing bioprocesses for cardiac cell therapy products (CTP) with improved functionality – supporting clinical trials.
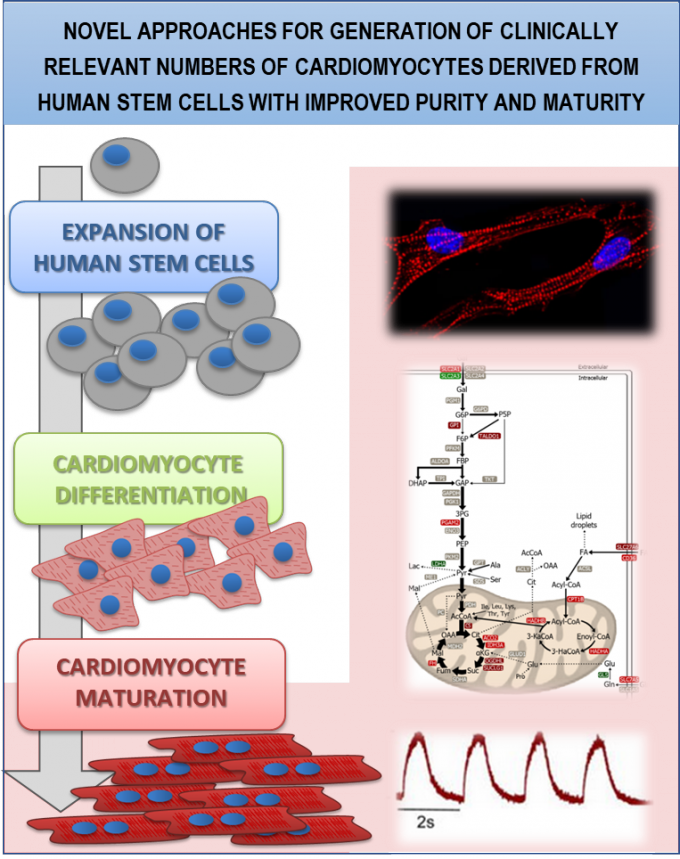
RG1 developed novel cell culturing strategies that recreate environmental conditions excelling growth and differentiation/maturation of human pluripotent and adult stem cells (ehPSC, hPSC-CM, hMSC, hCSC). In particular, we modulated culture medium composition to mimic the metabolic substrate usage by adult CMs in vivo, producing hPSC-CM with superior maturity and functionally developed (collaborations with Harvard Medical School, Murdoch Children’s Research Institute and CEDOC/NOVA-MS). Aiming at accelerating Cardiac CTP translation into the clinic, we integrated proteomics, transcriptomics, metabolomics and fluxomics as complementary analytical tools to bioprocess optimization and product characterization, with a strong emphasis on understanding the cardiac regenerative processes. Together with Tigenix/Coretherapix and CNB-CSIC, we implemented robust MS-based workflows for identity/potency assessment of hCSC, currently in clinical trial: NCT02439398.
2. GMP-compatible viral vectors design and production – molecular tools for advancing quality and quantity
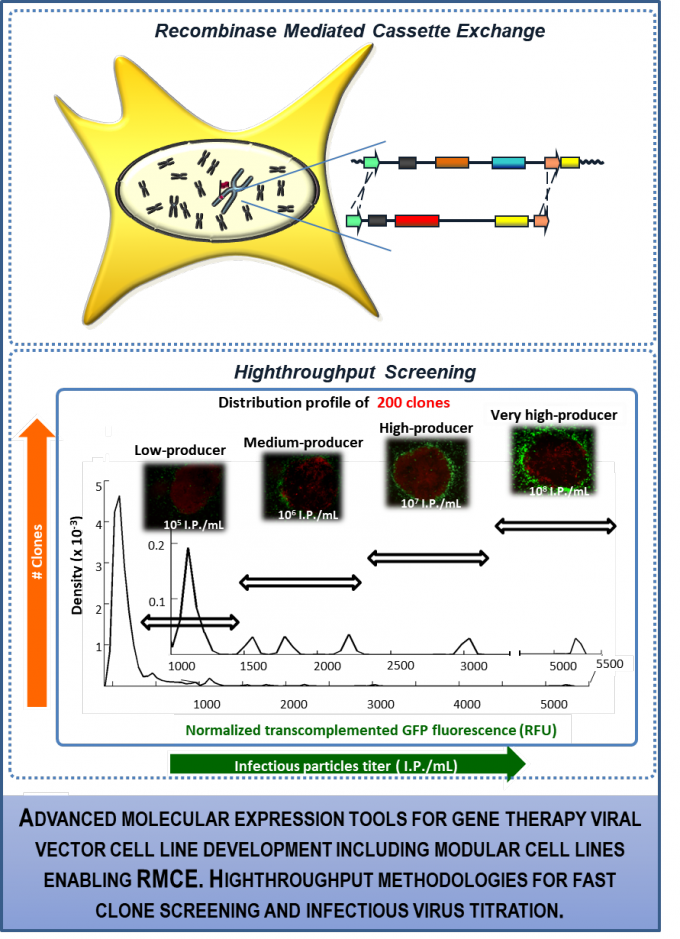
RG1 team has been developing work in gene and cell therapy supporting pre-clinical and clinical trials. By using expertise on recombinase-mediated cassette exchange, the team has developed novel gammaretrovirus packaging cell lines in the scope of immunotherapies, namely in CAR-T cell therapy (with UNUM Therapeutics). Additionally, through molecular design and mutagenesis, new lentiviral packaging expression vectors and cell lines were generated and used to establish a constitutive producer cell line (with Helmholtz) as well as pseudotyped AAVs to target Triple negative breast cancer (with Harvard Medical School). On a second line of work, RG1 has been developing novel cell-based methodologies to detect and quantify viruses and viral vectors. We developed the ‘Single step cloning screening’ technology (enabling functional detection and smart highthroughput screening of cells producing gammaretroviral or lentiviral vectors) and cell based sensors for the detection of label-free viruses (eg. adenoviral and lentiviral vectors).
3. Human Liver cell models to study liver-stage malaria and support drug discovery
RG1 expertise on development of advanced in vitro cell culture models, such as long-term 3D hepatic cell culture in bioreactors and on analytical tools for the evaluation of hepatic functionality were used to establish (in collaboration with IMM) an hepatic cell model of Plasmodial infection, to mimic the liver-stage of Malaria. Currently the model is being employed in target identification studies and medium-throughput drug discovery campaigns in collaboration with Merck – a patent has been filled.
Keywords: Stem Cells Bioengineering; Cell and Gene Therapy, Cell Products Characterization; Cell-based in vitro disease models
Latest Publications
Formas-Oliveira AS, Basílio JS, Rodrigues AF, Coroadinha AS (2020) Overexpression of ER Protein Processing and Apoptosis Regulator Genes in Human Embryonic Kidney 293 Cells Improves Gene Therapy Vectors Production. Biotechnology Journal 1900562.
Pais DA, Galrão PR, Kryzhanska A, Barbau J, Isidro IA, Alves PM (2020) Holographic Imaging of Insect Cell Cultures: Online Non-Invasive Monitoring of Adeno-Associated Virus Production and Cell Concentration. Processes 8(4): 487.
Vasconcelos e Sá J, Simão D, Terrasso AP, Silva MM, Brito C, Isidro IA, Alves PM, Carrondo MJT (2020) Unveiling dynamic metabolic signatures in human induced pluripotent and neural stem cells. PLOS Computational Biology 16(4): e1007780.
Moreira AS, Silva AC, Sousa MFQ, Hagner-McWhirterc A, Ahlénc G, Lundgren M, Coroadinha AS, ALves PM, Peixoto C, Carrondo MJT (2020) Establishing Suspension Cell Cultures for Improved Manufacturing of Oncolytic Adenovirus. Biotechnology Journal. 16:e1900411.
Guerreiro MR, Freitas DF, Alves PM, Coroadinha AS (2019) Detection and Quantification of Label-Free Infectious Adenovirus Using a Switch-On Cell-Based Fluorescent Biosensor. ACS Sensors 4(6):1654-1661